Case study: the hidden costs of investigator and site contracts
Creating a clinical trial budget is a difficult process; realizing belatedly that your budget may not reflect reality adds to the whiplash. With clinical trials increasingly being outsourced to third-party vendors and CROs (contract research organizations), sponsors remain several steps removed from the data they need to fully assess the scope and financial health of their studies.
Investigating hidden investigator costs
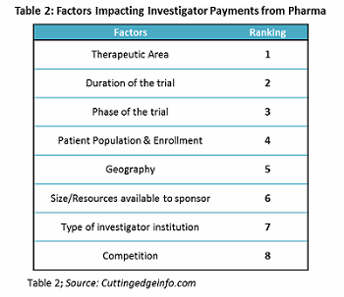
When embarking upon an upcoming study, a sponsor and a CRO will routinely build out and agree upon a trial budget which will be granular, detailed, and comprehensive. Yet it will also be only an estimate. When the CRO gets final confirmation, they will then proceed to negotiate and contract with sites, investigators, and other third parties in the interest of the study. These third-party costs, while budgeted for in the initial study agreement, may vary drastically from what was initially expected, due to factors such as cost differentials by therapeutic area, geography, enrollment, site mix, negotiating power, and fluctuations in fair market value for investigator compensation.
Source: Cuttingedgeinfo.com via Clinical Leader
The delta between the initial budget and what is contracted can often be a blind spot for sponsors. Typically, the CRO will not reforecast based on actuals, leaving the sponsor expecting one set of costs and potentially being hit with invoices that leave it scrambling to reassess. Given the variability and volatility in site contracts, the CRO views itself in a more project management-centered role, connecting the sponsor to resources, but not directly responsible for the financial trajectory of the study.
The difference between budget and actual contracts can be far beyond mere sticker shock: it can be material to the health of the study. If invoiced costs far exceed expectations, the runway of the trial (or of the sponsor itself) could be in jeopardy, forcing difficult and unanticipated choices on the sponsor.
Software supports sponsors
Earlier this year, our team onboarded a notable biotech client to the Auxilius FP&A platform. This client is a publicly traded entity with trials ongoing across specialized therapeutic areas, and it has dedicated accounting, finance, and ClinOps teams.
As part of implementation, Auxilius team members scour all possible financial documents, contracts, and data sources to provide a current and accurate view of the trial(s). During this phase, the team unpacked the client’s site contracts and realized the contracted amounts far exceeded budgeted investigator fees. The team quickly built out a new investigator forecast using these actuals and shared it with the client.
The differences were stark. As the site mix and enrollment data had coalesced, several high-cost geographies/sites had seen larger than expected enrollment, increasing total costs. Notably, several sites also had contracted costs that were triple or quadruple what had been budgeted per patient. As it stood in the final site contracts, the total contracted investigator costs amounted to nearly double the initially budgeted amount.
The Auxilius solution surfaced key insights:
- Site costs per patient ranged from 1– 4x anticipated per-patient cost
- One site had a six-figure salary contracted but not accounted for in budget
Unfortunately, sponsors often find themselves in similar situations when relying on CROs or third-party vendors for study-specific or financial information. While resource constraints across teams and functions may prevent every document and contract from being minutely scrutinized, there are several steps sponsors can take to help prevent blind spots and put themselves in the driver’s seat.
- Update your average per-patient spend as site contracts come in
In coordination across finance and ClinOps, sponsors should review site contracts whenever possible and adapt their expected per-patient spend based on what has been rubber stamped. This will serve as preliminary and directional guidance on what to expect as sites start to enroll patients.
- Ensure you're accounting for other costs if they are outsized at the site
When the Auxilius team delved into several site contracts further, they uncovered more unexpected expenses: in one, the six-figure salary had been negotiated but not accounted for by the CRO’s budget. If there are site-specific expenses, make sure your budget identifies and clarifies them.
- Update as enrollment happens (and you know about your site mix)
When sites go live and patients enroll, update your assumptions: for instance, if many patients are enrolling at a comparatively high-cost site, that will need to be reflected in your forecast. This process should continue throughout enrollment, giving you an ever-clearer view of the true per-patient cost.
Visibility is paramount
Clinical trials are essential for getting new therapeutics to market; having trials derailed by unexpected expenses is in no one’s interest. Uncovering and avoiding hidden costs takes an alignment of people, process, and technology, but it’s worth the effort. With scrutiny and software dedicated to the task, sponsors can set their studies up for success.
Interested in learning more about how our product can support your team?